License for Drugs (Medicine) Export
View Procedure
| Procedure Name | License for Drugs (Medicine) Export | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | Category : License Renewal Frequency of the License : Renewal is not required Issuing Ministry : Ministry of Health and Family Welfare
Incumbent Office : Name: Directorate General of Drug Administration
Address: 105-106, Motijheel C/A, Dhaka-1000, Bangladesh
E-mail: drugs@citech.net
Website: www.dgda.gov.bd
Issuance of license for Drugs (Medicine) Export
Process Steps
Process Map
General Information Legal Basis of the License: The Drugs Act, 1940, [Section -18(C);] The Nature of the License: The Drug Rules 1945 The purpose of the license: Pharmaceutical Sector Territorial Scope of the License: Operational License Eligibility Criteria to Obtain the License: National Information Availability: Holder of Manufacturing License of Medicine
| ||||||||||||||||||||||||
| Category | Directorate General of Drug Administration (DGDA) |
| Title | Description | Created Date | Updated Date | Issued By | 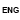 |
|---|---|---|---|---|---|
| Drugs Export License Application Form | The form is known as FORM 1O-A. An applicant needs to submit the form to the Directorate General of Drug Administration office to get the Drugs Export License. Please click the PDF mark to view or download the form in English. | 05-10-2015 | 09-10-2017 |
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| No results found. | |||||||